Some simple ones are they have a pH below 7 they turn blue litmus paper red they have a sour taste and they react with alkalis to form saltsOne interesting factor about acids is acid strength. Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products.
The concentration of the solution greatly affects the dissociation to form the hydrogen ion and the conjugate base acetate CH 3 COO At a concentration comparable to that in vinegar 10 M the pH is around 24 and only around 04 percent of the acetic acid molecules are.
. Exothermic reactions release energy into the surroundings so they usually feel hot. Now if we know the value for K a we can calculate the hydrogen ion concentration and therefore the pH. This makes acetic acid a monoprotic acid with a pKa value of 476 in aqueous solution.
Then all you have to do is to find the pH using. Learn more in this KS3 Chemistry guide from Bitesize. K a for ethanoic acid is 174 x 10-5 mol dm-3.
Remember that we want to calculate the pH of a buffer solution containing 010 mol dm-3 of ethanoic acid and 020 mol dm-3 of sodium ethanoate. When used for cleaning soap solubilizes particles and. Endothermic reactions are the opposite.
In a domestic setting soaps are surfactants usually used for washing bathing and other types of housekeepingIn industrial settings soaps are used as thickeners components of some lubricants and precursors to catalysts. You are already aware of the term acidsYou have learned about the physical and chemical properties of acids.

Ph O Level Secondary Chemistry Tuition
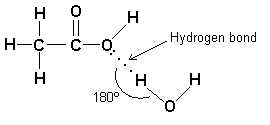
Solubility And Ph Of Ethanoic Acid

To Find The Ph Of The Samples By Using Ph Paper Universal Indicator Lab Work

0 Comments